Is oleander toxic?
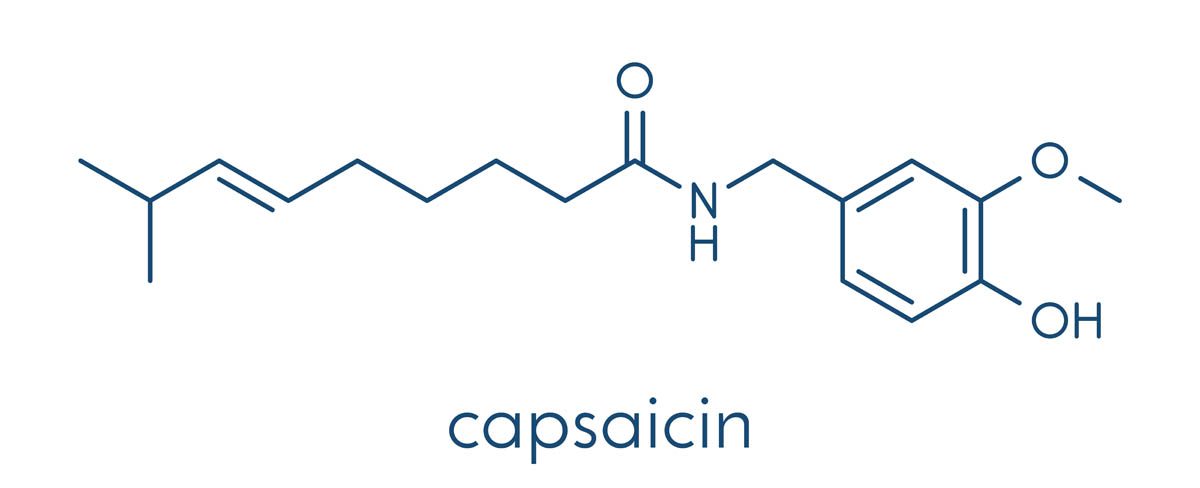
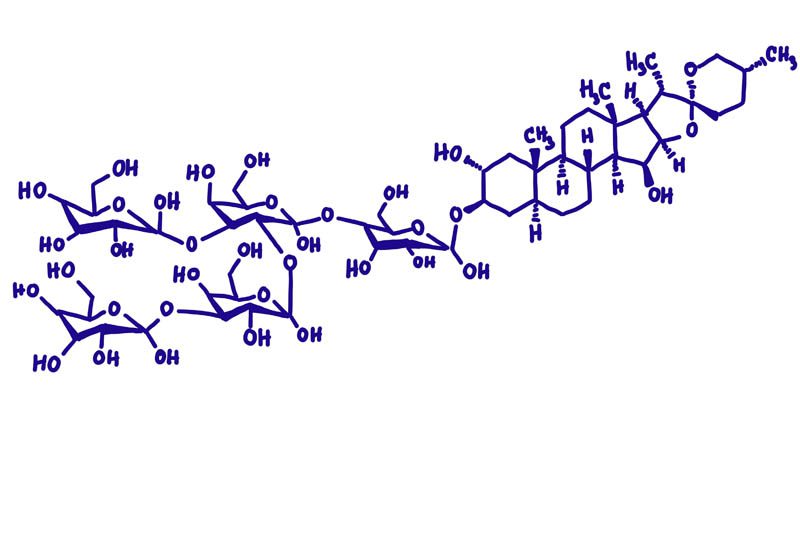
Nerium oleander, commonly referred to as ‘oleander‘, is a highly toxic plant native to the Mediterranean basin and Asia. The toxic properties are several cardiac glycosides collectively known as ‘cardenolides‘ including oleandrin, neriine, digitoxigenin, and digitoxin. These toxins disrupt the sodium-potassium pump (Na+/K+ ATPase pump) in the cell membrane. The sodium-potassium pump maintains the proper balance of sodium and potassium ions inside and outside the cell, which is critical for many cellular processes, such as nerve impulse transmission, muscle contraction, and nutrient uptake.
Cardiac glycosides are present in all parts of the oleander tree, including leaves, flowers, stems, roots and seeds. The highest concentration is found in the seeds, followed by the leaves. Dry oleander remains toxic and livestock have been poisoned by drinking water with the fallen leaves. Inhalation of smoke from burning oleander is also toxic.
Oleander poisoning ranks equal to mushroom poisoning for children’s hospital admissions in Australia and as little as one leaf can be fatal to an adult.
What is oleander?

| Botanical name |
Nerium oleander |
| Common names |
Oleander, Common oleander, Rose bay, Soland, Rose laurel |
| Family |
Apocynaceae |
| Plant type |
Shrub, Tree |
| Mature height |
2 – 6 metres (6.5 – 19.6 feet) |
| Bloom time |
Spring to autumn |
| Flower colour |
White, red, pink, yellow, orange, cream, purple |
| Leaf colour |
Green |
| Toxicity |
Toxic to humans, dogs, cats, livestock |
| Toxic properties |
Cardiac glycosides (oleandrin, neriine,
digitoxigenin, and digitoxin) |
Oleander is an ornamental shrub or small tree native to the Mediterranean, found in warm climates across the globe that is popular for its fragrant, showy flowers that bloom from late spring to autumn. The plant has long, narrow, leathery leaves that are arranged in whorls, and its five-lobed flowers grow in pink, red, white, cream, orange and purple.
Oleander is a popular ornamental plant due to its showy and fragrant flowers that bloom from late spring to fall, and its ability to tolerate a range of soil types and climatic conditions. The plant has long, narrow, and leathery leaves that are arranged in whorls, and its flowers are typically pink, red, or white and five-lobed. Oleander is also known for its use in traditional medicine, where it has been used to treat various ailments such as asthma, bronchitis, and stomach disorders.
In addition to its ornamental value, oleander has been used in traditional medicine to treat heart conditions, skin irritations and respiratory problems.
It is important for home gardeners to be aware of the plants in their garden and the potential risks some pose. As a pet owner and gardener, oleander is not a plant I would choose to grow due to its high degree of toxicity.
Identification

Leaves
The leaves are long, narrow, and lance-shaped (lanceolate), measuring between 10 – 15 cm long and 2.5 cm wide. Leaf colour is glossy green on the upper surface, with a smooth and leathery texture. The leaves are arranged in pairs or whorls along the branch.
Flowers
The trumpet-shaped flowers have a diameter of 3 – 6 cm and are borne in clusters at the end of the branches. Each flower has five loves that are often ruffled at the edges.
Oleander flowers are showy and trumpet-shaped, with a diameter of 1.5 to 3 inches. Each flower has five petal-like lobes that are often slightly ruffled at the edges. The flowers are borne in clusters at the end of branches and can range in color from white to various shades of pink and red, depending on the cultivar. The flowers typically bloom from late spring through the fall, with a peak bloom period in the summer. The plant also produces a slender, cylindrical seed pod that contains numerous small, feathery seeds.
Toxicity
The toxic properties are oleandrin, neriine, digitoxigenin, and digitoxin, collectively known as cardiac glycosides. Cardiac glycosides are a secondary metabolite that affects the function of the heart by inhibiting the activity of the Na+/K+ ATPase pump. The concentration of these toxins can vary depending on the age of the plant, time of year and environmental conditions. Of all the cardiac glycosides that occur in oleander, oleandrin is the most toxic.
Touching oleander is generally not harmful, however, always wash your hands thoroughly after handling any part of the plant.
Mechanism of action
Oleander contains cardiac glycosides, including oleandrin, which can interfere with the function of the Na+/K+ ATPase pump.
- Inhibition of Na+/K+ ATPase pump: The primary mechanism of action for oleander toxicity is the inhibition of the sodium-potassium ATPase pump (Na+/K+ ATPase) in cell membranes, particularly in cardiac myocytes (muscle cell). This pump plays a crucial role in maintaining the balance of sodium and potassium ions across the cell membrane, which is essential for generating and propagating electrical impulses in the heart.
- Disruption of the ion balance and electrical activity: Inhibition of the Na+/K+ ATPase pump causes sodium ions to accumulate in the fluid inside the cell (intracellular fluid), while potassium ions accumulate in the fluid outside the cell (extracellular fluid). This imbalance causes a depolarisation of the cell membrane (a change in the electrical potential across the cell membrane, resulting in a less negative or more positive potential difference between the inside and outside of the cell), which in turn, activates voltage-gated calcium channels, leading to an influx of calcium ions into the cells.
- Increased intracellular calcium and force of contraction: The increase in intracellular calcium levels enhances the contractile force of the heart (positive inotropic effect) which can lead to excessive workload on the heart and increased oxygen demand, potentially causing arrhythmias, heart failure, or ischemia.
Disruption of the normal electrical activity within the heart due to the altered ion balance can lead to various types of arrhythmias (abnormal heart rhythms) and conduction abnormalities. These disturbances can range from relatively mild, such as premature ventricular contractions (PVCs), to potentially life-threatening, such as ventricular tachycardia or fibrillation.
Severe poisoning can lead to neurological disorders as the toxins affect the balance of neurotransmitters in the brain. Altered ion balance interferes with the generation and propagation of electrical signals in neurons, leading to impaired nerve impulse transmission.
In addition to the toxic effects on the heart, oleander can also cause a variety of extracardiac symptoms due to its action on smooth muscle cells of the gastrointestinal tract.
What is the purpose of cardiac glycosides?
Cardiac glycosides are secondary metabolites (metabolites not involved in the growth or reproduction of the plant) that are produced to protect the plant against herbivory from animals and insects. Other plants known for their cardiac glycosides include foxglove (Digitalis purpurea), lily of the valley (Convallaria majalis), hawthorn (Crataegus spp.), squill (Urginea maritime), Christmas rose (Hellebore spp.) and cascabela (Cascabela thevetia).
How does oleander poisoning occur?
Poisoning may be accidental, or deliberate (attempted murder or suicide). Children and pets are at greater risk due to their curious nature while adults may mistake oleander for a non-toxic species.
Herbal remedies from unknown sources, or using unknown ingredients is another potential risk factor. Always buy supplements and remedies from reputable sources and know what’s in the product.
Burning oleander plant material can release toxic fumes as cardiac glycosides aren’t destroyed by heat.
Poisoning can also occur if food or drink is contaminated with oleander plant parts, such as using oleander branches for skewers, mistakenly using oleander leaves as a food wrap, or brewing tea from the leaves or flowers.
What are the symptoms of oleander poisoning?
Ingesting or contact with any part of the plant can lead to a host of symptoms affecting the cardiovascular, gastrointestinal and neurological systems. The severity of the symptoms can vary depending on the amount of toxin ingested and the individual’s susceptibility, but prompt recognition and treatment are critical for managing the toxic effects and improving the chances of recovery. The following list details some of the most common symptoms associated with oleander poisoning:
Gastrointestinal:
- Loss of appetite
- Nausea (possibly with blood)
- Vomiting
- Diarrhea
- Melena (dark, tarry stools caused by the presence of digested blood)
- Abdominal pain
Cardiac:
- Palpitations (irregular, rapid, or forceful heartbeats)
- Bradycardia (slower than normal heartbeat)
- Tachycardia (faster than normal heartbeat)
- Arrhythmias (abnormal heart rhythms)
- Chest pain
- Dyspnea (shortness of breath)
- Heart failure
- Sudden cardiac arrest
Neurological:
- Dizziness
- Headache
- Confusion
- Disorientation
- Altered mental status
- Seizures
- Coma (in extreme cases)
Dermal:
Dermal contact with oleander sap can cause skin irritation in some individuals. While dermal contact with oleander is generally less dangerous than ingestion or inhalation, it is still important to exercise caution when handling this plant. If you come into contact with oleander sap, wash the affected area thoroughly with soap and water to minimize irritation. If you develop a rash or severe skin reaction, consult a healthcare professional for appropriate treatment.
Use in medicine
Despite its toxic nature, the toxic compounds have potential medical uses.
- Cardiac applications: Oleander extracts have been historically used in small, controlled doses to treat heart failure and certain arrhythmias. The cardiac glycosides in oleander, exert positive inotropic effects (increasing the force of the heart’s contractions) and negative chronotropic effects (decreasing the heart rate) by inhibiting the sodium-potassium ATPase pump in heart cells, which increases intracellular calcium levels.
- Anticancer activity: Some compounds derived from oleander have been studied for their potential anticancer effects. One such compound is Anvirzel, a patented extract derived from oleander. Anvirzel has shown promising results in preclinical studies by inducing apoptosis (programmed cell death) in cancer cells. It is thought that the cardiac glycosides in Anvirzel may inhibit cancer cell growth by disrupting cellular metabolism and energy production. Clinical trials are ongoing to evaluate the safety and efficacy of Anvirzel for cancer treatment.
Treatment
There is no specific antidote to oleander toxicity. The goal of treatment is to decontaminate the GI tract to prevent further absorption and manage clinical signs.
- Gastrointestinal decontamination: If the poisoning occurred through ingestion and the patient presents within one hour of exposure, healthcare providers can induce vomiting to decontaminate the GI tract, this will be followed up with administration of activated charcoal (a highly porous form of carbon with a large surface area and strong adsorptive properties, commonly used to bind to and remove toxins) to bind to any remaining toxins and reduce their absorption in the gastrointestinal tract.
- Fluid therapy: Intravenous fluids can help maintain blood pressure and hydration, as well as support kidney function and assist in the elimination of toxins and correct electrolyte imbalances.
- Antiarrhythmic medications: If the patient experiences life-threatening arrhythmias due to oleander toxicity, healthcare providers may administer antiarrhythmic medications to help stabilize the heart rhythm.
- Digoxin-specific antibody fragments (Digibind or DigiFab): Healthcare providers may consider using digoxin-specific antibody fragments for severe toxicosis. This medication binds to and neutralises cardiac glycosides, thereby reversing their toxic effects on the heart. Although these antibody fragments were initially developed for digoxin poisoning (cardiac glycoside medication derived from the foxglove plant), they have shown effectiveness in treating severe oleander poisoning as well.
- Supportive care: Healthcare providers will continue to monitor and manage any symptoms or complications that arise from oleander poisoning, such as treating pain, nausea, or seizures, and providing respiratory support if necessary.
Safety
- Keep out of reach of children and pets: Make sure oleander plants are planted or stored in areas inaccessible to children, pets, or livestock. Puppies are like toddlers, and they love to explore their world. Unlike toddlers, they have no hands and everything goes into their mouths. As the current owner of a puppy, I have regularly watched her proudly bring branches and sticks into the house to play with. Educate children about the potential dangers of the plant and instruct them not to touch or ingest any part of it.
- Wear protective gear: Wear gloves, long sleeves, and eye protection when handling oleander.
- Wash hands and tools: After handling oleander, thoroughly wash your hands and any tools used to avoid accidental ingestion or contact with the toxic compounds.
- Avoid burning: Never burn oleander plant parts, as the smoke can release toxic compounds that may cause respiratory irritation or other harmful effects if inhaled.
- Be cautious with plant debris: Dispose of oleander clippings and plant debris carefully. The toxins can remain potent even in dried or dead plant material.
- Do not use for food preparation: Never use oleander plant parts or utensils that have come into contact with the plant for food preparation or consumption.
- Seek immediate medical attention: If you or someone you know ingests any part of an oleander plant or experiences symptoms of oleander poisoning (such as vomiting, diarrhea, irregular heartbeat, dizziness, or seizures), seek immediate medical attention.
How long does oleander poisoning last?
The duration of oleander poisoning symptoms varies on the amount of toxin ingested, the individual’s sensitivity to the toxin, and the overall health of the person affected. In general, symptoms of oleander poisoning last anywhere from a few hours to several days.
Initial symptoms such as nausea, vomiting, and abdominal pain may resolve within hours, however, more severe symptoms like cardiac issues, and coma may persist for a longer period. In some cases, it can take several days for the affected individual to fully recover.
Prompt medical treatment is crucial for managing oleander poisoning, as it can help alleviate symptoms and prevent complications. In severe cases, if left untreated, oleander poisoning can be fatal. It is essential to seek immediate medical attention if oleander poisoning is suspected.
Conclusion
- Oleander is a beautiful and hardy plant that contains a number of potent toxins known as cardiac glycosides.
- All parts of the plant are toxic to humans and animals upon ingestion or inhalation.
- Oleander poisoning can lead to a variety of symptoms, including gastrointestinal distress, cardiac arrhythmias, and even death in severe cases. While dermal contact with the plant may cause irritation in some individuals, the risk of toxicity is significantly lower than when ingested.
- Despite its toxic nature, oleander has been used in traditional medicine and continues to be a favourite among gardeners for its vibrant flowers and hardy nature.
Julia is a writer and landscape consultant from Wollongong with a love of horticulture. She had been an avid gardener for over 30 years, collects rare variegated plants and is a home orchardist. Julia is passionate about learning and sharing her knowledge of plant propagation and plant toxicology. Whether it’s giving advice on landscape projects or sharing tips on growing, Julia enjoys helping people make their gardens flourish.