What is soil pH?
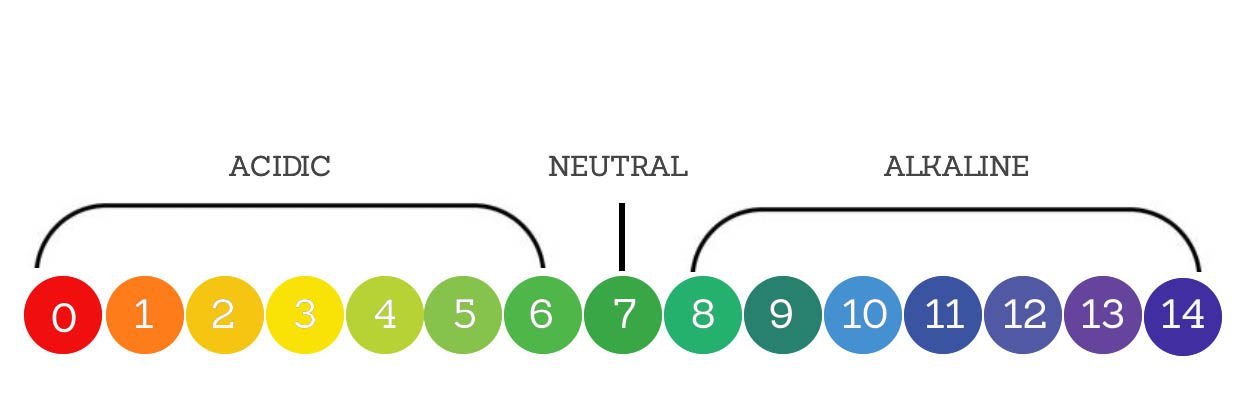
Soil pH is the acidity or alkalinity of soil which plays an important role in plant health. The pH scale ranges from 0 to 14. 7 is neutral pH, values below 7 indicate the soil is acidic, while values above 7 indicate that the soil is alkaline. Soil pH impacts the availability of nutrient uptake and the activity of soil microorganisms.
Low (acidic) pH soil has a high hydrogen ion concentration and high (alkaline) pH values have a low hydrogen ion concentration. Most plants prefer a neutral to slightly acidic pH range of 6.0 to 7.5. However, some plants have very specific preferences. For example, blueberries thrive in acidic soil of pH 4.5 to 5.5), while asparagus prefers alkaline soil of pH 7.0 to 8.0.
Various factors influence soil pH including rainfall, the type of minerals and organic matter present in the soil. Areas with high rainfall generally have more acidic pH as rainwater can leach away basic elements such as calcium and magnesium. Arid regions are typically more alkaline.
What does pH mean?
 The term “pH” stands for “potential of hydrogen” or “power of hydrogen.”It measures the concentration of hydrogen ions (H⁺) in a solution. The pH scale is logarithmic, which means that each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times more acidic than a pH of 6. Similarly, each pH value above 7 is ten times more alkaline than the next lower value.
The term “pH” stands for “potential of hydrogen” or “power of hydrogen.”It measures the concentration of hydrogen ions (H⁺) in a solution. The pH scale is logarithmic, which means that each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times more acidic than a pH of 6. Similarly, each pH value above 7 is ten times more alkaline than the next lower value.
- 7.0—neutral
- > 7.5—alkaline
- < 6.5—acidic (soil with pH less than 5.5 is strongly acidic)
How do hydrogen ions form in water?
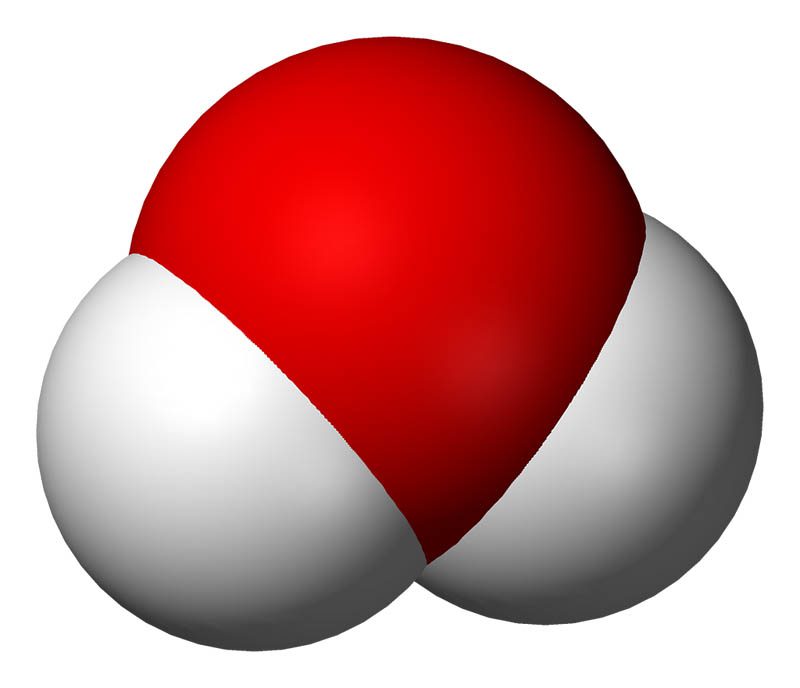
Oxygen and hydrogen are atoms, which can bond together to form a molecule. A water molecules are made up of two hydrogen atoms and one oxygen atom (H₂O) that are bonded together.
When a water molecule (H₂O) donates a proton, H⁺ (a hydrogen atom without its electron) to another water molecule, it becomes a hydronium ion (H₃O⁺). The receiving water molecule, which accepts the proton, then becomes a hydroxide ion (OH⁻). This happens during the self-ionisation of water.
This occurs due to the constant collisions between molecules in a liquid or gaseous state. This type of interaction is common in many chemical reactions.
Factors that determine soil pH
Most soils have a pH range between 3.5 and 10. There are several factors that influence the pH of soil:
- Parent material: The original rock material that formed the soil, called parent material, influences the initial pH of the soil. Rocks such as limestone create more alkaline soil, while granite can lead to more acidic soil.
- Rainfall: Areas with high rainfall generally have more acidic soils. This occurs because water can leach basic ions, such as calcium and magnesium, out of the soil, leaving behind more acidic ions like aluminium and iron.
- Plant and microbe activity: Certain plants and soil microbes can alter the soil pH. For example, legumes work with bacteria to fix nitrogen, which, in turn, makes the soil more alkaline. When plant material decomposes, organic acids can be produced, making the soil more acidic.
- Human activity: Fertilisers, lime, or sulfur, can significantly change soil pH. For example, applying lime (calcium carbonate) raises soil pH, making it more alkaline, while adding sulfur can lower the pH, making the soil more acidic.
Related: Pythium Infection in Plants
How does soil pH impact plants?
Soil pH levels play a significant role in a plant’s health and growth. When soil pH is too high or low, it can affect the availability of nutrients essential for plant growth.
Acidic soil:
Low (acidic) pH soil (high hydrogen ions) impacts the availability of phosphorous, calcium and magnesium.
- Phosphorous (P) can react with aluminium and iron in highly acidic soil to form iron phosphate and aluminium phosphate, both of which are insoluble, and therefore unavailable to plants.
- Calcium (Ca) and magnesium carry a positive charge (cations). In highly acidic soil, high levels of hydrogen ions that carry a positive charge are present. Hydrogen ions can outcompete calcium and magnesium for binding sites on negatively charged soil particles. This results in less calcium and magnesium available for plant uptake. High acidity can also increase calcium and magnesium leaching, further reducing their availability in the soil. Once displaced from the soil particles, calcium and magnesium ions become part of the soil solution. When rainfall or irrigation exceeds the soil’s ability to hold water, this excess water drains through the soil, carrying with it soluble nutrients, including the displaced calcium and magnesium.
- Aluminium (Al) is abundant in the earth’s crust and is normally locked up in minerals such as feldspars and clays. When soil pH drops below 5, these aluminium-containing minerals begin to dissolve, releasing aluminium ions (Al3+) into the soil solution which can be taken up by plant roots. High concentrations of Al3+ can damage root tips and limit the ability of the roots to take up water and nutrients.
- Manganese (Mn) is an essential micronutrient for plants but can be toxic in excess when present in excess. Under acidic conditions, the Mn2+ ion becomes highly available and can be taken up in large amounts by plant roots. This can lead to an accumulation of manganese in the plant tissues, interfering with the plant’s metabolic processes, particularly photosynthesis.
Alkaline soil:
In alkaline (low hydrogen ions) soil iron, manganese, boron, copper, and zinc become less soluble. On the other hand, nutrients like calcium and magnesium can become more available.
- Iron (Fe) and manganese (Mn) can oxidise (combine with oxygen), to form iron (III) oxide and manganese (IV) oxide. These compounds are less soluble and hence less available to plants as they form a type of rust in the soil, which the roots are unable to absorb.
- Phosphorus (P) can combine with calcium (Ca) to form calcium phosphate, a compound that is not easily dissolved in water and thus not readily available for plants to absorb.
- Iron (Fe), copper (Cu), and zinc (Zn) can form insoluble chelates (a compound that contains a ligand bonded to a central metal atom), that are not available to most plants.
- When the soil pH is too high (alkaline), these enzymes produced by beneficial bacteria, fungi and other microbes may not function effectively, reducing the microbes’ ability to metabolise nutrients and reproduce.
- Sodium (Na), lead (Pb), copper (Cu), cadmium (Cd), nickel (Ni) and zinc (Zn) are more soluble and thus more bioavailable at high pH levels. This can be harmful to acidophilic microbes, nitrogen-fixing bacteria as well as other soil-dwelling bacteria, fungi, and archaea.
How to test soil pH
Testing soil pH is important to ensure plants are grown under optimal conditions to ensure they thrive. The good news is that pH testing is easy and cheap, starting from $10.00 for a pH meter.
pH meter:
pH meters are readily available at garden centres for a few dollars. They consist of two metal prongs that are inserted into the soil, an internal electric reference electrode and circuitry, a battery compartment and a display screen.
pH meters with metal prongs measure the voltage potential between the prongs when they are inserted into the soil. This is created due to the difference in hydrogen ion concentrations between the soil and a reference electrode inside the meter. The meter converts this voltage difference into a pH reading based on the relationship between voltage and pH.
How to use:
pH meters are simple to use. Just insert the prongs into the soil and the needle will show you the pH of the soil. When you are finished, wipe any soil debris from the prongs and rinse in distilled water. Distilled water has a neutral pH of 7, which means it won’t interfere with the pH reading of the next sample.
Vinegar and baking soda test:
The vinegar and baking soda test is a rudimentary and qualitative method to get a rough idea of whether the soil is acidic, alkaline, or neutral. This test is not precise and should not be relied upon for accurate pH measurements.
- Alkalinity: White vinegar can test the alkalinity of a soil sample. Pour a small amount of white vinegar onto a sample of soil. If it starts to fizz or bubble, the soil is alkaline, with a pH above 7.
- Baking soda: Take a moist soil sample and place it in a small container. Sprinkle a small amount of baking soda onto the soil. If it fizzes or bubbles, it indicates that the soil is acidic, with a pH below 7.
If neither tests produce any fizzing or bubbling, the soil has a pH of around 7.
pH paper:
A soil sample is collected from multiple spots and a soil: water suspension is made with distilled water to create a slurry. The most common ratio is 1:5 (soil:water). The mixture is allowed to settle, which takes between 30-60 minutes. Once this has occurred, the soil will have settled, leaving a layer of coloured (supernatant) water at the top. A pH paper strip is dipped into the supernatant and then removed. The soaked pH strip will change colour based on the acidity or alkalinity of the soil solution. This is compared to a colour chart provided to determine the pH.
Professional testing services:
A professional soil testing service can provide the most accurate result. The most common method is the 1:5 (soil:water) suspension method. This method involves creating a soil-water mixture, allowing it to settle, and then measuring the pH of the water. Here’s a step-by-step description of the process:
A small amount of soil is collected from multiple spots within an area to get a representative sample. This soil is then mixed with distilled water in a ratio of 1:5 (soil:water). So, for every part of soil, you add five parts of water. The sample is mixed thoroughly. After mixing, the soil-water suspension is allowed to settle for a specific period. This could be anywhere from half an hour to several hours, depending on the specific protocol being followed. After the soil settles, a pH meter is used to measure the pH of the water. The electrode of the pH meter is inserted into the supernatant (the clear liquid above the settled soil), and the pH reading is taken.
Correcting pH balances
Soil pH can be adjusted to suit the requirements of specific plants. Adding lime to the soil can raise its pH (make it more alkaline) while adding sulfur or peat moss can lower soil pH. Commercially available products are also available to help adjust soil pH. Gardeners and farmers need to test and, if necessary, adjust the pH of their soil to ensure that it is suitable for the plants they are growing, as it can significantly affect plant health and yield.
Effects of soil pH on flower colour

Most gardeners have heard about the effects of soil pH on hydrangeas. Acidic soil below 6 pH produces blue flowers and alkaline pH produces pink. Aluminium ions (Al3+) are easily absorbed by the plant roots and are transported through the vascular system too various parts of the plant, including the petals.
Anthocyanins are a type of water-soluble pigment present in the vacuoles of flowers, fruits and vegetables. Vacuoles are membrane-bound organelles located in plant cells that serve various functions, including storage of pigments and other compounds. Anthocyanins are responsible for the wide range of colours we see in the plant kingdom.
Hydrangea petals contain an anthocyanin known as delphinidin (Dp). Delphinin is a purple-coloured plant pigment belonging to the cyanidin-based anthocyanins. Not only is delphinin responsible for the purple hue in a range fo plants (including hydrangea), it is also an antioxidant.
Delphinidins are pH-sensitive. In acidic environments, the vacuoles of petal cells decrease, stabilising the molecular structure of delphinidin, and resulting in the characteristic blue colour. This process is known as ‘metal ion-induced colouration‘.
In alkaline soil, there is a decrease in the availability of aluminium ions and an increase in calcium and magnesium ions. These ions compete with aluminium ions for binding sites within the plant, reducing the uptake of aluminium by the roots. The resulting reduction or absence of aluminium ions leads to a chemical modification of the anthocyanin molecules, resulting in the formation of pink or red petals.
Hydrangeas flowers may be the most well-known plants impacted by soil pH, but other plants are also affected, including azaleas, rhododendrons, blueberries, lilacs and clematis.
Julia is a writer and landscape consultant from Wollongong with a love of horticulture. She had been an avid gardener for over 30 years, collects rare variegated plants and is a home orchardist. Julia is passionate about learning and sharing her knowledge of plant propagation and plant toxicology. Whether it’s giving advice on landscape projects or sharing tips on growing, Julia enjoys helping people make their gardens flourish.




